
Mobile Health POC Diagnostics for Malaria
NIAIDContract No. 75N93021C00031; 07/19/2021-07/18/2024
Project Officer: Dr. Deirdre Joy, Ph.D. • Principle Investigator: Espoir Kyubwa
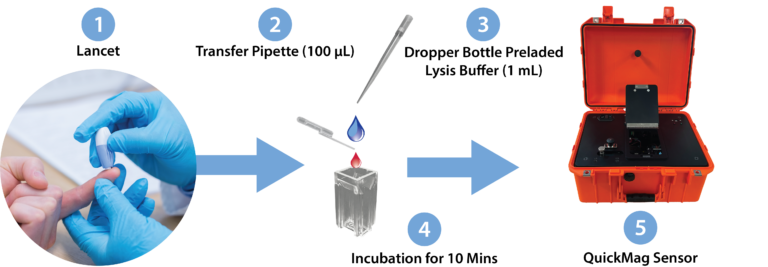
The QuickMag system consists of a low-cost rugged magneto-optical sensing subsystem to detect and quantify the presence of hemozoin in plasma samples. Blood samples are collected by a simple venipuncture and prepared for the reader using a disposable reagent and pipette pack. One pack used per sample.

System Features
- Highly-sensitive malaria screening tool for low-resource settings.
- Based on the detection of hemozoin, produced by all malaria parasites and present in infected blood.
- Uses a phone-based application to process the test and provide high-confidence malaria parasitemia diagnosis.
- Provides a low cost, rapid, easy-to-use, POC malaria test with the sensitivity needed to reliably diagnose early-stage and asymptomatic infections.
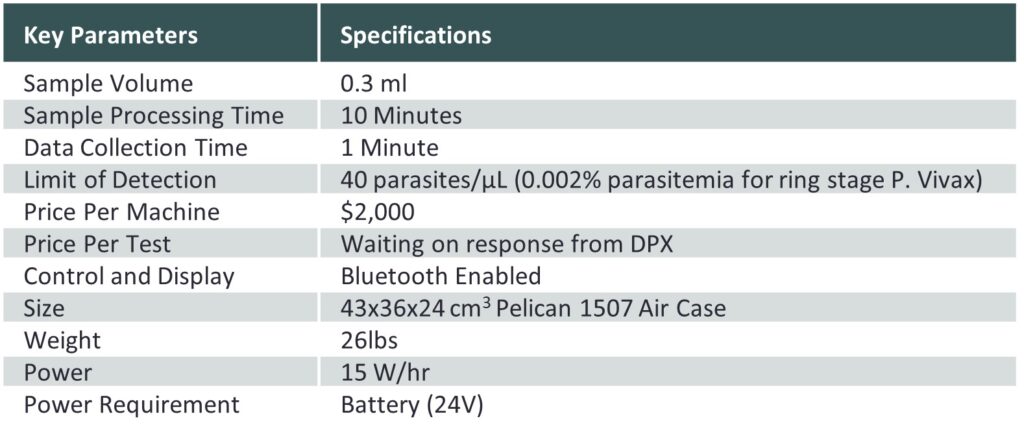
QuickMag System Specs
Program Goals
- Optimize the Quick-Mag sample processing subsystem rapid, automated, and efficient purification of hemozoin from whole blood.
- Demonstrate robustness of the Quick-Mag sample processing on clinically-relevant Plasmodium species. .
- Develop quality control and manufacturing processes for Quick-Mag sample processing subsystem.
- Optimize the Quick-Mag sensor subsystem, resulting in a rapid, sensitive, battery-operated, field-portable, affordable malaria detection device.
- Enhance the Quick-Mag sensor software to enable rapid control and data display using the device dashboard or Bluetooth device.
- Evaluate the performance (LOD, specificity, sensitivity) of the integrated Quick-Mag system.
- Evaluate performance of the integrated Quick-Mag system on de-identified clinical samples in malaria endemic area.
- Obtain WHO prequalification
- Explore Commercialization of the Quick-Mag system
Current Status
- Finalized design and assembly of Quick-Mag field deployable unit in Pelican Case.
- Started Testing of QuickMag field deployable unit vs benchtop unit.
- Lyophilization of calibration standard ongoing for 3 months (90+ days).
- Lysis buffer stable for 2 months, being tested up to 6 months.
- Signed NDA with GMI Solutions (US CMO), sent RFQ.
Next Steps
- Vibration travel testing of field unit (UCR deployment)
- Complete stability tests of the lysis buffer and calibration standards.
- Deploy the Quick-Mag unit out in the field and test malaria positive clinical samples in 1st clinical study, improvement of the unit and 2nd deployment for gathering data for FDA pre-submission.
- Plan for minor software and UI development for end user.








